- Research
- Open access
- Published:
The associations of psychopathology and metabolic parameters with serum bilirubin levels in patients with acute-episode and drug-free schizophrenia: a 5-year retrospective study using an electronic medical record system
BMC Psychiatry volume 24, Article number: 403 (2024)
Abstract
Background
The oxidative system plays an important role in the pathogenesis of schizophrenia. Inconsistent associations were found between hyperbilirubinemia and psychopathology as well as glycolipid metabolism in patients with schizophrenia at different episodes. This current study aimed to examine these associations in patients with acute-episode and drug-free (AEDF) schizophrenia.
Methods
This is a retrospective study using 5 years of data from May 2017 to May 2022 extracted from the electronic medical record system of Chaohu Hospital of Anhui Medical University. Healthy controls (HCs) from the local medical screening center during the same period were also included. Participants’ data of the bilirubin levels [total bilirubin (TB), conjugated bilirubin (CB), unconjugated bilirubin (UCB)], glycolipid metabolic parameters and the score of the Brief Psychiatric Rating Scale (BPRS) were collected.
Results
A total of 1468 case records were identified through the initial search. After screening, 89 AEDF patients and 100 HCs were included. Compared with HCs, patients had a higher CB level, and lower levels of glycolipid metabolic parameters excluding high density lipoprotein-cholesterol (HDL-C) (all P < 0.001). Binary logistic regression analyses revealed that high bilirubin levels in the patients were independently associated with higher total and resistance subscale scores of BPRS, a higher HDL-C level, and lower total cholesterol and triglyceride levels (all P < 0.05).
Conclusion
Bilirubin levels are elevated in patients with AEDF schizophrenia. Patients with high bilirubin levels have more severe psychopathology and relatively optimized glycolipid metabolism. In clinical practice, regular monitoring of bilirubin levels in this patient population should be carried out.
Introduction
Approximately 1% of the worldwide population is affected by schizophrenia [1], while the China Mental Health Survey showed that the lifetime prevalence of schizophrenia in China is 0.6% [2]. Schizophrenia is often combined with hyperbilirubinemia, which is usually considered a sign of liver or hemolytic diseases [3, 4]. The prevalence of hyperbilirubinemia is about 25% in patients with schizophrenia, which is 2–3 times higher than in the general population [5].
Bilirubin is the end product of heme metabolism in human red blood cells, and comes in two forms: conjugated bilirubin (CB) and unconjugated bilirubin (UCB) [6]. On the one hand, a study reported that bilirubin had a strong antioxidant effect at a concentration below 100 nanomoles per liter (nM), which can protect nerve cells from oxidative stress damage [7]. On the other hand, high bilirubin levels can exhibit significant pro-oxidant and neurotoxic effects, ultimately leading to neuronal necrosis or apoptosis [8, 9]. The dysfunction of the oxidative system may be involved in the pathogenesis of schizophrenia [10]. In both animals and humans, higher bilirubin levels have been shown to be strongly associated with a greater risk of schizophrenia and more severe psychiatric symptoms. For example, an animal study showed that Gunn rats, a model of hyperbilirubinemia, have remarkably schizophrenic-like behaviors [11], and a cohort study with 21-year follow-up found that newborns with hyperbilirubinemia had an increased risk of developing schizophrenia in adulthood [12]. Moreover, Miyaoka et al. reported that levels of biopyrrins, oxidative products of bilirubin, are positively correlated with the score of Brief Psychiatric Rating Scale (BPRS) in patients with chronic schizophrenia [13]. Additionally, previous studies found that there were significant differences in UCB levels among three groups (schizophrenia > schizoaffective disorder > bipolar disorder), which indicates that UCB may be a potential biomarker in schizophrenia [14,15,16]. Therefore, given the complex mechanisms of action of bilirubin, it is important to reveal the characteristics of bilirubin and its relationship with psychopathology in patients with schizophrenia.
In the general population, bilirubin levels were negatively correlated with total cholesterol (TC), triglyceride (TG), and fasting blood glucose (FBG) levels, which have been repeatedly confirmed in previous meta-analysis, cohort study, and national survey [17,18,19]. Similarly, in patients with schizophrenia, CB level was negatively correlated with the levels of TG and FBG, which has been shown to be a valid predictor of risk for metabolic syndrome (MetS) [20].
The differences in bilirubin levels in patients with schizophrenia across studies may be due to the heterogeneity of the subjects studied (e.g. gender, intake of antipsychotics, and different periods of illness). For example, in a previous cross-sectional study, bilirubin levels were higher in males with schizophrenia than in females [21]. Christian et al. found that patients with schizophrenia had significantly lower bilirubin levels after 2 and 4 weeks of antipsychotic treatments [22]. Moreover, both case-control and prospective studies showed that patients with acute-phase schizophrenia had higher bilirubin levels than those with remission-phase schizophrenia [23, 24]. Therefore, this current study aimed to examine (1) the levels of bilirubin in patients with acute-episode and drug-free (AEDF) schizophrenia and their associations with psychopathology, and (2) the associations between bilirubin levels and glycolipid metabolism parameters in patients with AEDF schizophrenia.
Methods
Study design and participants
This is a retrospective study using 5 years of data from May 2017 to May 2022 extracted from the electronic medical record system of Chaohu Hospital of Anhui Medical University. Patients were included, if they met the following criteria: (1) Han Chinese, aged between 18 and 65 years; (2) diagnosed as schizophrenia, based on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) using a structured Clinical Interview, and were in an acute-episode as defined by BPRS score ≥ 40 [25]; (3) without antipsychotic treatments for the last 1 month [26]. Patients were excluded who had (1) any other psychiatric disorders; (2) organic brain diseases, hypertension, diabetes, hyperlipidemia, immune system diseases, hepatic diseases or other serious physical diseases; (3) or were pregnant or breastfeeding women; (4) recently used any anti-inflammatory drugs. The HCs were recruited from the local medical examination in the same period, who were Han Chinese, aged between 18 and 65 years, and had no personal or family history of psychiatric diseases.
In accordance with the Declaration of Helsinki, all participants and their guardians signed an electronic informed consent via smartphone. The protocol of this retrospective study was approved by the Medical Ethics Committee of Chaohu Hospital of Anhui Medical University on 13 October 2022 (202210-kyxm-015).
Data collection measurements
All participants’ socio-demographic and clinical data were collected through the electronic medical record system, including age (years), sex (male and female), age of onset (years), duration of illness (months). Body mass index (BMI) was calculated as weight (kg)/height (m)2. Blood samples from patients were collected between 06:00 and 07:00 AM after an overnight fast. And bilirubin [total bilirubin (TB), CB, UCB], and glycolipid metabolism parameters [TC, TG, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), FBG] were measured. Psychiatric symptoms of the patients included in this study were assessed using the Chinese version of the 18-item BPRS [27, 28]. Each item was evaluated on a seven-point scale, ranging from “0 = not present” to “7 = extremely severe”, with a higher total score indicating more severe symptoms [29]. The scale consists of five subscales: affect subscale (items 1, 2, 5, and 9), negative symptoms subscale (items 3, 13, 16, and 18), positive symptoms subscale (items 4, 8, 12, and 15), resistance subscale (items 10, 11, and 14), and activation subscale (items 6, 7, and 17) [30].
Statistical analysis
The continuous and categorical variables were described as Mean ± standard deviation (SD) and frequency distributions (%), respectively. The Kolmogorov–Smirnov one-sample test was used to test the normal distribution of continuous variables. Patients were divided into low and high serum bilirubin groups using the 50th percentile of total TB, CB, and UCB, respectively [20]. Socio-demographic and clinical characteristics were compared between groups using independent samples t-test, Mann-Whitney U test, and Chi-square test as appropriate. Binary logistic regression models (Forward: LR) were used to examine the independent correlates associated with bilirubin levels, with bilirubin levels (categorical) as the dependent variables, and the variables which were significant in univariate analyses (P < 0.05) as the independent variables, as well as age and sex as the control variables. The correlations between bilirubin levels (after ln-transformed) and other clinical data, as well as metabolic parameters in the patients group were examined with Pearson or Spearman correlation analyses. Then multivariate linear regression models (Forward: LR) were used to examined any significant correlations (P < 0.05) in correlation analyses. Statistical Product and Service Solutions (SPSS) version 23.0 (SPSS incorporated, Chicago, Illinois, United States of America) was used for statistical analyses. The P - values were set as two-tailed α = 0.05.
Results
Case record selection and participant characteristics
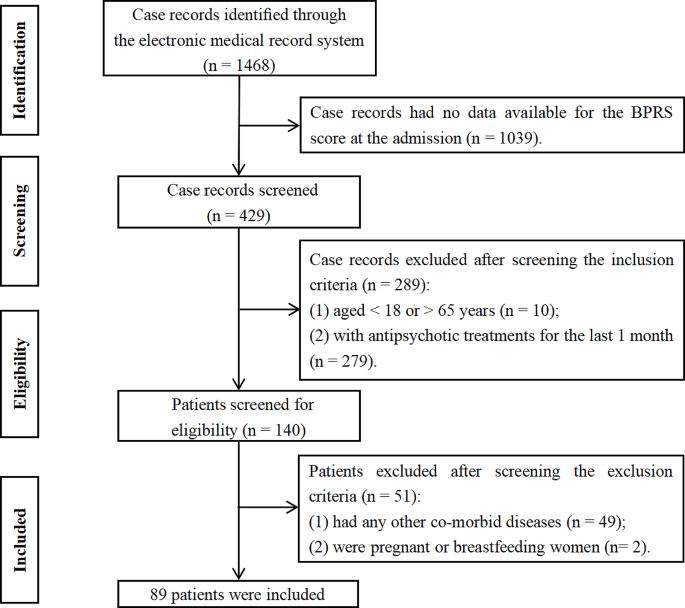
A total of 1,468 potentially relevant case records were initially identified (Fig. 1). After screening according to the inclusion (1,328 excluded) and exclusion (51 excluded) criteria, 89 patients with AEFD schizophrenia were included. And 100 HCs from the local medical examination were also included. In this study, the mean age of patients was 38.92 years (SD = 13.75), and 42.7% were male. The mean age of HCs was 35.35 years (SD = 6.95), and 42.0% were male. There were significant differences in BMI between groups (all P < 0.001) (Table 1).
Comparisons between patients with schizophrenia and healthy controls
As shown in Table 1, the patients with schizophrenia had a higher CB level, and lower levels of TC, TG, LDL-C, and FBG than HCs. Analysis of covariance (ANCOVA) showed that differences in levels of CB (F(1,189) = 20.223, P < 0.001), TC (F(1,189) = 10.380, P = 0.002), TG (F(1,189) = 6.648, P = 0.011), LDL-C (F(1,189) = 18.098, P < 0.001), and FBG (F(1,189) = 6.053, P = 0.015), between the two groups remained significant after controlling for BMI.
Comparisons between patients with low and high bilirubin levels
As shown in Table 2, the patients in the high TB group had a lower TG level, and a higher total score of BPRS, and higher scores on negative symptoms and resistance subscales than those in the low TB group (all P < 0.05). The patients in the high CB group had lower levels of TC, TG, LDL-C, a higher total score of BPRS, and scores on resistance and activation subscales than those in the low CB group (all P < 0.05). In addition, the patients in the high UCB group had a lower TG level, a higher HDL-C level, and a higher total score of BPRS, and a higher resistance subscale score than those in low UCB group (all P < 0.05).
Factors associated with high bilirubin levels (categorical)
Binary logistic regression (model 1: BPRS total score, or model 2: BPRS subscale scores involved in the regression models, respectively) analyses revealed that the high TB level (categorical) was significantly associated with a lower TG level [model 1: Odds Ratio (OR) = 0.25, 95% Confidence Interval (CI) = 0.07–0.90; model 2: OR = 0.25, 95% CI = 0.07–0.87], a higher total score of BPRS (OR = 1.09, 95% CI = 1.03–1.16) and a higher resistance subscale score (OR = 1.17, 95% CI = 1.04–1.32). The high CB level was significantly associated with a lower TC level, a higher total score of BPRS and a higher resistance subscale score (all P < 0.05). And the high UCB level was significantly associated with a higher HDL-C level, a higher total score of BPRS, and a higher resistance subscale score (all P < 0.05) (Table 3).
Independent correlates of bilirubin levels (continuous)
There were several correlations between socio-demographic and clinical variables with bilirubin levels (continuous) in patients (Table 1S). Multivariate linear regression analyses showed that the TB level was negatively associated with levels of TG (β = -0.26, P = 0.025), and was positively associated with resistance subscale score (β = 0.05, P < 0.001). The CB level was negatively associated with levels of TG and FBG, and was positively associated with total score of BPRS (all P < 0.05). The UCB level was negatively associated with TG level, and was positively associated with resistance subscale score (all P < 0.05) (Table 2S).
Discussion
In this study, we examined the peripheral bilirubin levels and their associations with psychopathology and glycolipid metabolism parameters in patients with AEDF schizophrenia. Consistent with the findings of two recent studies conducted in patients with acute-episode schizophrenia, we found that patients with AEDF schizophrenia had a higher CB level than HCs [23, 31]. This may be due to the fact that patients with acute onset schizophrenia are in a state of high oxidative stress, and sustained oxidative stress induces rupture of the erythrocyte cell membrane, which increases serum bilirubin levels [32]. Since CB is formed by the conversion of UCB in the liver, a significant elevated CB level may reflect an increased overall bilirubin level. In turn, elevated UCB levels induce reactive oxygen species (ROS) and nitric oxide (NO) production by neuroglia cells, leading to further enhancement of oxidative stress [33]. In a previous study, drug-naive, first-episode patients with schizophrenia had significantly higher levels of UCB, compared to HCs [34]. A new insight suggests that we can distinguish schizophrenia from schizoaffective and bipolar disorders based on the degree of UCB elevation [35, 36]. However, there were no differences in UCB levels between patients with schizophrenia and HCs in this study. On the contrary, in a recent study, Huang et al. reported that there was no statistical difference in bilirubin levels between patients with first-episode schizophrenia and HCs [37]. Even more, some studies conducted in different countries (South Korea, China, and Norway) found varying degrees of reduction in serum bilirubin levels in patients with schizophrenia compared with HCs [21, 38, 39]. In this case, reduced bilirubin levels may indicate a defect in the antioxidant defense system of patients with schizophrenia or a sustained oxidative depletion during the chronic course of this disease [40, 41].
In this study, we also found some associations between bilirubin and psychopathology in patients with AEDF schizophrenia. First, bilirubin levels were positively associated with the total score of BPRS. A clinical study found that increased oxidative metabolites of bilirubin are correlated with higher scores of BPRS in schizophrenia, and higher scores of Hamilton depression scale (HAMD) in depression [42]. These findings suggest that higher bilirubin levels are associated with exacerbated psychotic states, possibly as a result of bilirubin-induced neurotoxic effects and pro-inflammatory responses, as well as alterations in brain structures. For example, high bilirubin levels result in a rapid increase in extracellular glutamate concentrations, which ultimately leads to neuronal and oligodendrocyte damage [43]. In addition, by inducing the secretion of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), bilirubin provokes mitochondrial swelling, and inhibits the formation of myelin, which aggravates the patient’s psychiatric symptoms [44,45,46]. Neuroimaging studies found that patients with schizophrenia combined with hyperbilirubinemia have an enlarged cerebrospinal fluid cavity and enhanced signal in areas such as the frontotemporal cortex and the limbic system and basal ganglia [47,48,49].
Second, we found that bilirubin levels were positively associated with the BPRS resistance subscale score, which is usually defined by hostility, uncooperativeness and suspiciousness [30]. One Portuguese study [24] found that a positive association between UCB level with disturbing and aggressive behaviors in patients with schizophrenia and schizoaffective, which bring a new insight into the novel role of UCB level as a biomarker for psychomotor agitation. This was confirmed by the improvement in psychomotor agitation with decreasing bilirubin levels during a subsequent treatment. In patients with hyperbilirubinemia, elevated oxygen radicals or oxidative stress markers are associated with an increased risk of aggressive behaviors [50, 51]. Sustained oxidative stress can lead to reduced neuronal membrane fluidity, ion channel inactivation and demyelination, which may result in brain connectivity and impulse control disorders [52, 53]. A meta-analysis and the United States National Survey showed that the prevalence of aggressive behavior in patients with schizophrenia and bipolar disorder was 33.3% and 12.2%, respectively, both much higher than in the general population [54, 55]. Therefore, it is necessary to monitor bilirubin levels, which can objectively and accurately assess the risk of aggression and hostility in patients with severe mental illness. Third, a study conducted in unmedicated patients with schizophrenia found that bilirubin levels were positively correlated with the excitement component score of Positive and Negative Syndrome Scale (PANSS) [22]. Similarly, we also found a significant correlation between a higher CB level and a higher activation subscale score of BPRS, which is usually defined by excitement, tension, and mannerisms–posturing. However, the correlation disappeared after adjusting for the other BPRS subscale scores. Our interpretation is that the high CB level may be an indicator of a higher activation score, but may not be independent of the other BPRS subscale scores. In clinical evaluation, the use and dissemination of biomarkers that can provide a mode of clinical monitoring are the future frontiers in the evaluation of schizophrenia and related spectrum disorders. In therapy, anti-inflammatory drugs were considered an effective and safe adjunctive therapy for improving the symptoms of schizophrenia [56]. For example, as an anti-inflammatory drug, minocycline can reduce oxidative stress and protect the brain from bilirubin neurotoxicity [57].
Overall, we also found that bilirubin levels were negatively associated with levels of FBG, TG, and TC, and were positively associated with HDL-C level in patients with AEDF schizophrenia. Of note, we observed differences in glycolipid metabolic parameters in patients with different hyperbilirubinemia subtypes. Specifically, in the logistic regression analysis model, patients with a higher TB level had a lower TG level, those with a higher CB level had a lower TC level, and those with a higher UCB level had a higher HDL-C level; In the linear regression model, CB, but not UCB levels, were negatively correlated with the FBG level. The differences were also observed in the general population and schizophrenia patients in previous cross-sectional and prospective studies [58, 59]. Bilirubin is a lipid-soluble substance, and different types of bilirubin bind differently to albumin. Compared to UCB, CB may be more readily separated from albumin to protect patients from metabolism disorders [17]. Regarding the mechanism of action of bilirubin on glycolipid metabolism, it has been found that bilirubin can reduce glucose and lipid accumulation by increasing insulin sensitivity and intracellular glucose uptake, and activating the aryl hydrocarbon receptor (AhR) signaling pathway [60, 61]. A meta-analysis found that the overall prevalence of MetS in patients with schizophrenia was 32.5%, and the prevalence in unmedicated patients was also as high as 20.2% [62]. Notably, the risk of cardiovascular death in patients with MetS was 1.6 times higher than in those without, and 3.2 times higher than in the general population [63, 64]. As a valid predictor of MetS risk, bilirubin has potential use in monitoring and assessing the risk of cardiovascular events and death in patients with schizophrenia.
In this study, there are several limitations. First, due to methodological limitations, including cross-sectional study design, no patients with medicated schizophrenia or other severe mental disorders (e.g. schizoaffective and bipolar disorders), we were unable to make comparisons of bilirubin levels between subgroups [65]. Second, factors potentially associated with bilirubin levels, such as smoking, the use/abuse of caffeine, and pro re nata treatment (e.g. psychotherapy and counseling intervention) before blood sampling, were not examined. Finally, we have to acknowledge the importance of excluding pseudo- or organic schizophrenia, such as Gilbert syndrome by genetic testing, encephalitis by cerebrospinal fluid testing, and encephalic anomaly by electroencephalography and magnetic resonance imaging [24, 66]. Therefore, our results should be considered preliminary.
Conclusion
In summary, patients with AEDF schizophrenia had higher bilirubin levels, especially CB level, compared with HCs. Patients with high bilirubin levels have an exacerbated psychotic state with high risk of aggression, hostility and excitement; and have relatively optimized glycolipid metabolism with lower TG, TC and FBG levels and higher HDL-C levels. In clinical practice, regular monitoring of bilirubin levels is necessary to objectively and comprehensively assess psychopathology and the risk of glycolipid metabolism disorders in patients with schizophrenia. Given that our findings are concerning but preliminary, longitudinal studies are needed to further explore whether management of peripheral bilirubin could improve the prognosis of this patient population.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Hasan A, Falkai P, Lehmann I, Gaebel W, Schizophrenia. Dtsch Arztebl Int. 2020;117(24):412–19.
Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–24.
Hamoud AR, Weaver L, Stec DE, Hinds TD. Jr. Bilirubin in the Liver-Gut Signaling Axis. Trends Endocrinol Metab. 2018;29(3):140–50.
Zipursky A, Bhutani VK, Odame I. Rhesus disease: a global prevention strategy. Lancet Child Adolesc Health. 2018;2(7):536–42.
Müller N, Schiller P, Ackenheil M. Coincidence of schizophrenia and hyperbilirubinemia. Pharmacopsychiatry. 1991;24(6):225–8.
Vítek L, Jirásková A, Malíková I, Dostálová G, Eremiášová L, Danzig V, et al. Serum bilirubin and markers of oxidative stress and inflammation in a healthy Population and in patients with various forms of atherosclerosis. Antioxid (Basel). 2022;11(11):2118.
Doré S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96(5):2445–50.
Bortolussi G, Shi X, Ten Bloemendaal L, Banerjee B, De Waart DR, Baj G, et al. Long-Term effects of Biliverdin Reductase a Deficiency in Ugt1(-/-) mice: impact on Redox Status and Metabolism. Antioxid (Basel). 2021;10(12):2029.
Bianco A, Dvořák A, Capková N, Gironde C, Tiribelli C, Furger C, et al. The extent of Intracellular Accumulation of Bilirubin determines its Anti- or pro-oxidant effect. Int J Mol Sci. 2020;21(21):8101.
Lambert B, Semmler A, Beer C, Voisey J. Pyrroles as a potential biomarker for oxidative stress disorders. Int J Mol Sci. 2023;24(3):2712.
Hayashida M, Hashioka S, Hayashida K, Miura S, Tsuchie K, Araki T, et al. Low serum levels of fibroblast growth factor 2 in Gunn rats: a Hyperbilirubinemia Animal Model of schizophrenic symptoms. CNS Neurol Disord Drug Targets. 2020;19(7):503–08.
Dalman C, Cullberg J. Neonatal hyperbilirubinaemia–a vulnerability factor for mental disorder? Acta Psychiatr Scand. 1999;100(6):469–71.
Miyaoka T, Ieda M, Hashioka S, Wake R, Furuya M, Liaury K, et al. Analysis of oxidative stress expressed by urinary level of biopyrrins and 8-hydroxydeoxyguanosine in patients with chronic schizophrenia. Psychiatry Clin Neurosci. 2015;69(11):693–8.
Pommerening Dornelles E, Gama Marques J, Ouakinin S. Unconjugated bilirubin and schizophrenia: a systematic review. CNS Spectr. 2019;24(6):577–88.
Gama Marques J, Pedro I, Ouakinin S. Unconjugated bilirubin and acute psychosis: a five years retrospective observational and controlled study in patients with schizophrenia, schizoaffective and bipolar disorders. Int J Psychiatry Clin Pract. 2019;23(4):281–85.
Gama-Marques J, Tinoco I, Pedro I, Leote F, Silva R, Ouakinin S. Unconjugated bilirubin and acute schizophrenia: a probable correlation? Actas Esp De Psiquiatria. 2017;45(2):79–88.
Liang C, Yu Z, Bai L, Hou W, Tang S, Zhang W, et al. Association of Serum Bilirubin with metabolic syndrome and non-alcoholic fatty liver disease: a systematic review and Meta-analysis. Front Endocrinol (Lausanne). 2022;13:869579.
Zhang X, Meng Z, Li X, Liu M, Ren X, Zhu M, et al. The association between total bilirubin and serum triglyceride in both sexes in Chinese. Lipids Health Dis. 2018;17(1):217.
Seyed Khoei N, Wagner KH, Sedlmeier AM, Gunter MJ, Murphy N, Freisling H. Bilirubin as an indicator of cardiometabolic health: a cross-sectional analysis in the UK Biobank. Cardiovasc Diabetol. 2022;21(1):54.
Karadag F, Sengul CB, Enli Y, Karakulah K, Alacam H, Kaptanoglu B, et al. Relationship between serum bilirubin levels and metabolic syndrome in patients with Schizophrenia Spectrum disorders. Clin Psychopharmacol Neurosci. 2017;15(2):153–62.
Pae CU, Paik IH, Lee C, Lee SJ, Kim JJ, Lee CU. Decreased plasma antioxidants in schizophrenia. Neuropsychobiology. 2004;50(1):54–6.
Widschwendter CG, Rettenbacher MA, Kemmler G, Edlinger M, Baumgartner S, Fleischhacker WW, et al. Bilirubin concentration correlates with positive symptoms in patients with schizophrenia. J Clin Psychiatry. 2016;77(4):512–6.
Lu Z, Wen T, Wang Y, Kan W, Xun G. Peripheral non-enzymatic antioxidants in patients with schizophrenia: a case-control study. BMC Psychiatry. 2020;20(1):241.
Gama Marques J, Ouakinin S. Clinical profile in schizophrenia and schizoaffective spectrum: relation with unconjugated bilirubin in a prospective and controlled study with psychopathological and psychosocial variables. CNS Spectr. 2020;25(6):782–89.
El Kissi Y, Samoud S, Mtiraoui A, Letaief L, Hannachi N, Ayachi M, et al. Increased Interleukin-17 and decreased BAFF serum levels in drug-free acute schizophrenia. Psychiatry Res. 2015;225(1–2):58–63.
Zhou X, Wang X, Li R, Yan J, Xiao Y, Li W, et al. Neutrophil-to-lymphocyte ratio is independently Associated with severe psychopathology in Schizophrenia and is changed by Antipsychotic Administration: a large-scale cross-sectional retrospective study. Front Psychiatry. 2020;11:581061.
Overall JE, Gorham DR. The brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799–812.
Zhang M, Wang Z. Brief Psychiatric Rating Scale Chinese version (BPRS-CR) application notes. Shanghai Archives Psychiatry. 1983;03:130–33.
Overall JE, Hollister LE, Pichot P. Major psychiatric disorders. A four-dimensional model. Arch Gen Psychiatry. 1967;16(2):146–51.
Shafer A. Meta-analysis of the brief psychiatric rating scale factor structure. Psychol Assess. 2005;17(3):324–35.
Rasool M, Malik A, Saleem S, Ashraf MAB, Khan AQ, Waquar S, et al. Role of oxidative stress and the identification of biomarkers Associated with thyroid dysfunction in Schizophrenics. Front Pharmacol. 2021;12:646287.
Glen AI, Glen EM, Horrobin DF, Vaddadi KS, Spellman M, Morse-Fisher N, et al. A red cell membrane abnormality in a subgroup of schizophrenic patients: evidence for two diseases. Schizophr Res. 1994;12(1):53–61.
Silva SL, Vaz AR, Barateiro A, Falcão AS, Fernandes A, Brito MA, et al. Features of bilirubin-induced reactive microglia: from phagocytosis to inflammation. Neurobiol Dis. 2010;40(3):663–75.
Wang S, Yuan X, Pang L, Song P, Jia R, Song X. Establishment of an assistive diagnostic model for schizophrenia with oxidative stress biomarkers. Front Pharmacol. 2023;14:1158254.
Marques JG. Unconjugated bilirubin in Schizophrenia/Schizoaffective Disorder Spectrum: an overlooked and underestimated biomarker candidate. Prim Care Companion CNS Disord. 2020;22(2):1902525.
Pradeep JR, Acharya MS, Radhakrishnan R, Srinivasan K. Elevated unconjugated bilirubin in Schizophrenia compared to bipolar affective disorder. Prim Care Companion CNS Disord. 2019;21(4):19m02448.
Huang K, Tang Y, Chen Z, Ding S, Zeng H, Zhao Y, et al. Comparison of hematological parameters between First-Episode Schizophrenia and Anti-NMDAR Encephalitis. Front Cell Dev Biol. 2022;10:895178.
Xu H, Wei Y, Zheng L, Zhang H, Luo T, Li H, et al. Relation between unconjugated bilirubin and peripheral biomarkers of inflammation derived from complete blood counts in patients with Acute Stage of Schizophrenia. Front Psychiatry. 2022;13:843985.
Solberg DK, Refsum H, Andreassen OA, Bentsen H. A five-year follow-up study of antioxidants, oxidative stress and polyunsaturated fatty acids in schizophrenia. Acta Neuropsychiatr. 2019;31(4):202–12.
Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018;63(1):68–78.
Yin XL, Jia QF, Zhang GY, Zhang JP, Shirao T, Jiang CX, et al. Association between decreased serum TBIL concentration and immediate memory impairment in schizophrenia patients. Sci Rep. 2019;9(1):1622.
Miyaoka T, Yasukawa R, Yasuda H, Shimizu M, Mizuno S, Sukegawa T, et al. Urinary excretion of biopyrrins, oxidative metabolites of bilirubin, increases in patients with psychiatric disorders. Eur Neuropsychopharmacol. 2005;15(3):249–52.
Brites D. The evolving landscape of neurotoxicity by unconjugated bilirubin: role of glial cells and inflammation. Front Pharmacol. 2012;3:88.
Vodret S, Bortolussi G, Jašprová J, Vitek L, Muro AF. Inflammatory signature of cerebellar neurodegeneration during neonatal hyperbilirubinemia in Ugt1 (-/-) mouse model. J Neuroinflammation. 2017;14(1):64.
Feng J, Li M, Wei Q, Li S, Song S, Hua Z. Unconjugated bilirubin induces pyroptosis in cultured rat cortical astrocytes. J Neuroinflammation. 2018;15(1):23.
Vaz AR, Falcão AS, Scarpa E, Semproni C, Brites D. Microglia susceptibility to free bilirubin is age-dependent. Front Pharmacol. 2020;11:1012.
Wake R, Miyaoka T, Tsuchie K, Kawakami K, Nishida A, Inagaki T, et al. Abnormalities in MRI signal intensity in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia. Aust N Z J Psychiatry. 2009;43(11):1057–69.
Miyaoka T, Yasukawa R, Mihara T, Mizuno S, Yasuda H, Sukegawa T, et al. Fluid-attenuated inversion-recovery MR imaging in schizophrenia-associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome). Eur Psychiatry. 2005;20(4):327–31.
Miyaoka T, Seno H, Itoga M, Inagaki T, Horiguchi J. Structural brain changes in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome): a planimetric CT study. Schizophr Res. 2001;52(3):291–3.
Tobore TO. On the neurobiological role of oxidative stress in Alcohol-Induced Impulsive, aggressive and suicidal behavior. Subst Use Misuse. 2019;54(14):2290–303.
Patki G, Atrooz F, Alkadhi I, Solanki N, Salim S. High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neurosci Lett. 2015;584:308–13.
Gorlova A, Svirin E, Pavlov D, Cespuglio R, Proshin A, Schroeter CA, et al. Understanding the role of oxidative stress, neuroinflammation and abnormal myelination in Excessive Aggression Associated with Depression: recent input from mechanistic studies. Int J Mol Sci. 2023;24(2):915.
Coccaro EF, Lee R, Gozal D. Elevated plasma oxidative stress markers in individuals with intermittent explosive disorder and correlation with aggression in humans. Biol Psychiatry. 2016;79(2):127–35.
Corrigan PW, Watson AC. Findings from the National Comorbidity Survey on the frequency of violent behavior in individuals with psychiatric disorders. Psychiatry Res. 2005;136(2–3):153–62.
Li W, Yang Y, Hong L, An FR, Ungvari GS, Ng CH, et al. Prevalence of aggression in patients with schizophrenia: a systematic review and meta-analysis of observational studies. Asian J Psychiatr. 2020;47:101846.
Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med. 2019;49(14):2307–19.
Zhang F, Chen L, Jiang K. Neuroinflammation in Bilirubin Neurotoxicity. J Integr Neurosci. 2023;22(1):9.
Li XH, Lin HY, Guan LY, Peng H, Wen MM, Cao YQ, et al. Direct bilirubin levels and risk of metabolic syndrome in healthy Chinese men. Biomed Res Int. 2017;2017:9621615.
Fu J, Wang Q, Zhang L, Liu J, Wang G. Serum bilirubin level is increased in metabolically healthy obesity. Front Endocrinol (Lausanne). 2021;12:792795.
Vitek L, Hinds TD Jr., Stec DE, Tiribelli C. The physiology of bilirubin: health and disease equilibrium. Trends Mol Med. 2023;29(4):315–28.
Bates EA, Kipp ZA, Martinez GJ, Badmus OO, Soundarapandian MM, Foster D, et al. Suppressing hepatic UGT1A1 increases plasma bilirubin, lowers plasma urobilin, reorganizes kinase signaling pathways and lipid species and improves fatty liver disease. Biomolecules. 2023;13(2):252.
Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–18.
Guembe MJ, Fernandez-Lazaro CI, Sayon-Orea C, Toledo E, Moreno-Iribas C. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13-year prospective study in the RIVANA cohort. Cardiovasc Diabetol. 2020;19(1):195.
Hayes JF, Marston L, Walters K, King MB, Osborn DPJ. Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014. Br J Psychiatry. 2017;211(3):175–81.
Gama Marques J, Arantes-Gonçalves F. A perspective on a possible relation between the psychopathology of the Schizophrenia/Schizoaffective Spectrum and Unconjugated Bilirubin: a longitudinal protocol study. Front Psychiatry. 2018;9:146.
Marques JG. Organic psychosis causing secondary Schizophrenia in one-Fourth of a cohort of 200 patients previously diagnosed with primary Schizophrenia. Prim Care Companion CNS Disord. 2020;22(2):19m02549.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Clinical Key Specialty Project Foundation (P. R. China), and the Natural Science Foundation of Anhui Province, China (Number: 2108085MH275) and the Scientific Research Project of Anhui Higher Education Institutions (Number: 2022AH050671).
Author information
Authors and Affiliations
Contributions
HL and LX were responsible for the design and direction of the study. YT, CY, LL, XZ and HF were responsible for the collection, analysis and interpretation of the data. Drafting of the manuscript was done by YT. HL and LX were responsible for critical revision of the manuscript. All the authors revised the final version for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
In accordance with the Declaration of Helsinki, all participants and their guardians signed an electronic informed consent via smartphone. The protocol of this retrospective study was approved by the Medical Ethics Committee of Chaohu Hospital of Anhui Medical University on 13 October 2022 (202210-kyxm-015).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tian, Y., Yang, C., Liu, L. et al. The associations of psychopathology and metabolic parameters with serum bilirubin levels in patients with acute-episode and drug-free schizophrenia: a 5-year retrospective study using an electronic medical record system. BMC Psychiatry 24, 403 (2024). https://doi.org/10.1186/s12888-024-05862-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-024-05862-5