- Research
- Open access
- Published:
A systematic review and meta-analysis of studies on screening for mild cognitive impairment in primary healthcare
BMC Psychiatry volume 22, Article number: 97 (2022)
Abstract
Background
Cognitive disorders and dementia have an important effect on individual independence and orientation. According to the Alzheimer's Disease International (ADI) 75% of people with dementia are not diagnosed; this may be as high as 90% in some low- and middle-income countries. This systematic review and meta-analysis aimed to identify the test performance of screening tools and compare them pairwise. The findings of our study can support countries in planning to establish and care for mild cognitive impairment in primary health centers.
Methods
Medline (PubMed), Scopus, Cochrane, Dare, All EBM Reviews, CRD (OVID), and Proquest were searched from 2012 to November 2021. The risk of bias was assessed through the QUADAS-2 instrument. Given the high heterogeneity between studies, a random-effects model was used to calculate the pooled effect sizes for diagnostic accuracy measures (sensitivity, specificity, and area under curve indices). I2 test was used for assessing heterogeneity and predefined subgroup analyses were performed using participants’ age, country’s income, and sample size of studies.
Results
A systematic search identified 18,132 records, of which, 20 studies were included in the quality assessment, and six were included in quantitative analysis. None of the studies had examined the feasibility or efficiency of mass screening. According to a pairwise comparison, IQCODE, AD8 and GPCOG showed equal or better diagnostic performance relative to the MMSE in terms of sensitivity and specificity. The random-effect model for the MMSE showed the pooled sensitivity equal to 0.73 (95% CI 0.57–0.90), the pooled specificity equal to 0.83 (95% CI 0.75—0.90), and the pooled AUC equal to 0.88 (95% CI 0.83–0.93).
Conclusion
Several benefits have been attached to short tests making them a suitable choice for use in primary healthcare settings. Considering factors such as accuracy, time of application, ease of scoring, and utilization charges, tests such as IQCODE, AD8, and GPCOG or appropriate combination with counterpart tools seem to be good alternatives to the use of the MMSE in primary care.
Background
Cognitive disorders and dementia have an important effect on individual independence and orientation. Alzheimer’s is characterized by impaired memory and dysfunction; it is one of the areas of aphasia, apraxia, amnesia, and dysfunction, which has a significant impact on individual and social functioning [1]. According to the World Alzheimer Report, over 55 million people worldwide live with dementia, and this number is expected to increase to 78 million by 2030. According to the mentioned report, 75% of people with dementia are not diagnosed; this may be as high as 90% in some low- and middle-income countries [2].
Additional research by the National Institute on Aging (NIA) at the National Institutes of Health (NIH) and the Alzheimer's Association (NIA-AA) highlighted modernizing concept in Alzheimer’s disease diagnosis [3]. The research groups introduced Alzheimer’s disease in a continuum with three discrete phases including preclinical, Mild Cognitive Impairment (MCI), and dementia. They suggested that Alzheimer’s disease (AD) is a pathophysiological construct similar to other diseases such as diabetes and osteoporosis. By using biomarkers, a clinical specialist might detect the disease in a person based on symptoms [4]. However, physicians are less likely to be able to diagnose cognitive disorders by formal examining or performing daily visits [5], therefore, up to 76% of patients are diagnosed only in moderate or severe dementia [6,7,8]. Early diagnosis of cognitive impairment can give patients and their families the opportunity to receive care in the early stages of the disease; this will lead to a better prognosis and improve living standards. Although early detection of cognitive impairment cannot halt the onset of the disorder, and existing treatments cannot reverse the course of the disease, the health, psychological, and social benefits of early detection are important enough to make a screening program worthwhile [9]. Werner et al. [10] conducted a systematic review to investigate dementia diagnosis disclosure among the patients and their families. Based on their findings, most studies have been positive about the disclosure of the disease. The patients' families have acknowledged that they were initially skeptical about the disease disclosure, then they later adapted it. Awareness of the diagnosis has led to better planning and preparation for the future.
There has been a growing interest among researchers and health systems for the early identification of people at risk of developing dementia. In fact, early accurate diagnosis of AD is a major global health priority [11]. The global action plan of the World Health Organization (WHO) on the public health response to dementia targets at least 50% of countries to diagnose 50% of the estimated number of people with dementia by 2025 [2]. The US Preventive Services Task Force (USPSTF) in its last update, reported that there was insufficient published evidence of better clinical outcomes as a result of routine screening for cognitive impairment in older adults. However, the Task Force recognized that the use of cognitive assessment tools can increase the detection of cognitive impairment [12]. Subsequently, the Patient Protection and Affordable Care Act (PPACA) in the United States recommended early diagnosis of cognitive impairment during the annual wellness visit. The workgroup developed ten recommendations for improving the early detection and care for dementia, concerning the implementation of cognitive screening practice in personalized healthcare [13]. According to the principals of Annals Wellness Visits (AWV), the early detection process is likely to occur in a primary care setting by using brief screening tests (taking a minimum time to administer), used by non-physician practitioners. Therefore, it is necessary to have easy-to-score, quick, open access, and sensitive tests to identify people with dementia in primary healthcare [14]. In recent years, systematic reviews and meta-analyses have attempted to identify diagnostic accuracy of both comprehensive and brief instruments for cognitive impairment and Alzheimer’s [15, 16]. Most of them have examined cognitive screening measures in secondary or tertiary care settings where the practice is run by physicians or neuropsychologist experts. The test performance of screening tools has not been widely assessed in the literature. In the study by Pelegrini et al. [15], diagnostic strategies in primary healthcare settings have been examined across low and middle-income countries. In spite of the short time interval of literature search (2013 to 2018), the study has only reported a sort of diagnostic criteria for screening tests’ performance and compared it among countries from different income streams. However, the gap of suitable instruments for use in primary healthcare settings has still been remained questionable. Lin et al., in an updated systematic review, attempted to address the benefits, harms, and diagnostic accuracy of brief screening instruments to detect cognitive impairment in community -dwelling older adults [16]. In spite of their conclusion in favor of the benefits of using brief instruments, they have not recognized empirical evidence on screening to improve decision-making. Considering the importance of early diagnosis for cognitive impairment as well as the consensus on primary care setting as the best start setting for assessment, our systematic review and meta-analysis aimed to identify test performance of screening tools and compare them pairwise. The findings of our study can support countries in planning to establish dementia care in primary health care centers.
Methods
The present systematic review was conducted in accordance with the preferred report items for systematic review and meta-analysis studies (PRISMA) [17]. The systematic review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database with the code CRD42020156638.
Inclusion and exclusion criteria
All English original studies including a) screening early detection of cognitive disorders in a primary care setting, b) using short questionnaires (according to the Alzheimer Association, the questionnaires that take less than 5 min to administer), c) and reporting sensitivity, specificity, positive and negative predictive values, and AUC measures for diagnostic tests and d) screening mild dementia were searched. The exclusion criteria were: a) studies that only examined the characteristics of diagnostic methods, b) or evaluated patient or provider’s opinion about the instruments, c) studies applied laboratory markers or imaging techniques to diagnose a particular type of dementia or Alzheimer's disease.
Data sources and search strategy
Databases including Medline (PubMed), Scopus, Cochrane, Dare, All EBM Reviews, Center for Research and Dissemination (CRD) via OVID, and Proquest were searched from the beginning of 2012 to November 2021. A search strategy is presented below for PubMed. A supplementary search across the references list and citations of included studies were also performed in Google Scholar to find related articles.
(TITLE-ABS-KEY (dementia OR Alzheimer OR "Cognitive Disorders" OR "Cognitive impairment" OR "Cognition Disorders" OR "cognitive decline" OR "cognitive loss") AND TITLE-ABS-KEY (screening OR "Early detection" OR "early diagnosis")) AND PUBYEAR > 2012 AND PUBYEAR < 2021.
Selection of studies
The study selection was independently done by two authors (LK and LJ). Any disagreement was resolved by the systematic review consultant (HS) or the clinical consultant (MF). After eliminating duplicates in the reference management software (EndNote) and manually (sorting by the title and year of the study), the titles and abstracts of the studies were screened according to the inclusion criteria. At this stage, screening programs were identified and studies that met the exclusion criteria were excluded. For the studies without the original article, the authors contacted the corresponding author (send an email or message in www.researchgate.net). If the reply message was not received after sending the message, the article was removed.
Data extraction
An Excel form was designed by the research team then administered to gather information about the author, year, country, population and place of the study, sample size, index, and reference test, reported outcome, and cut-off point. Data were independently extracted by (LK) and (AM) and sent to the (LJ) step by step for review and approval.
Risk of bias and quality assessment
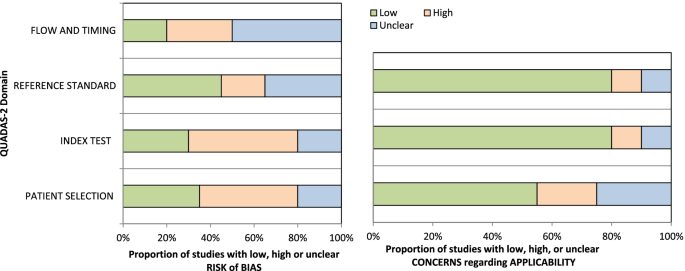
In order to assess the risk of bias in the studies, The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tools were used [17]. This tool has four domains of patient selection (three questions), index test (two questions), reference test (two questions), flow, and time (four questions). The probability of bias existence is reported in three levels of bias: low, uncertain, and high. Concerns about the usability of each domain are also reported in three forms: low, high, and unspecified. In fact, the purpose of this question is to evaluate the ability of the domain to answer the research question. In order to evaluate the quality of the studies, a software program designed by the QUADAS group was used. In this program, questions of each domain are listed, which by entering studies and evaluating them, the program allows the researcher to produce graphs and evaluation results in the form of excel tables. The risk of bias was assessed by LK and AM. In cases where clinical or epidemiological consultation was required, cases were raised and resolved with consulting professors (HS and MF). For minimizing biases and increasing reliability, selecting the studies for this systematic review was conducted through dual revision by two researchers. Cohen’s Kapa coefficient statistic was used for reporting the agreement.
Outcome measurement criteria
The outcome of interest consisted of the diagnostic accuracy indices of the screening tests, including sensitivity, specificity, or data that could be used to derive these values.
Summary of study findings and statistical analysis
In order to evaluate the accuracy of diagnostic screening tools, sensitivity and specificity of indices and reference tests were compared and reported in terms of study number and sample size. Given the high heterogeneity between studies, a random-effects model was used to calculate the pooled specificity, sensitivity, and AUC. I2 test was used for assessing heterogeneity and predefined subgroup analyses were performed using participants’ age, country’s income, and sample size of studies. The data were analyzed using STATA version 14 (STATA Corp, College Station, TX, USA). P-values of less than 0.05 were considered statistically significant. Publication bias test was conducted by funnel plot analysis.
Ethical considerations
The present study has been approved in Tabriz University of Medical Sciences (NO. IR.TBZMED.VCR.REC.1398.139).
Results
Studies characteristics
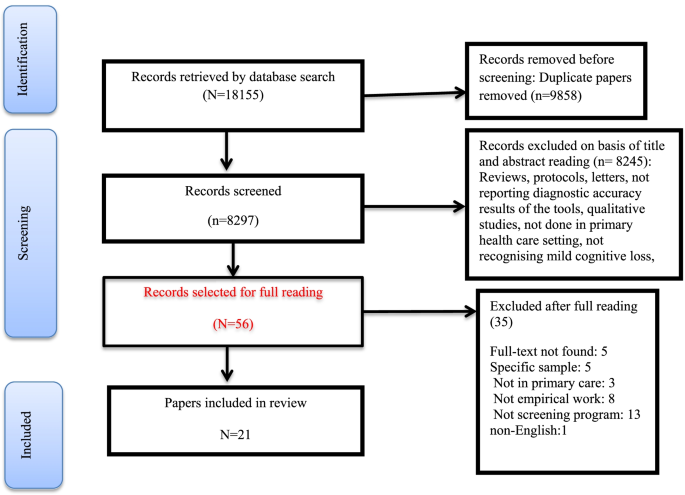
Systematic search identified 18,155 records, of which 9,858 articles were duplicates, and 8,245 records were not relevant which were excluded at initial screening of title and abstracts. After reviewing the title and abstract of the studies, 56 original articles were selected for the study. Of these, 35 studies were excluded because of not having eligible criteria. Finally twenty-one studies met the inclusion criteria for the systematic review and were included in the qualitative evaluation (Fig. 1). Characteristics of the studies were presented in Table 1, share of countries from the 21 final studies including Australia (n = 1) [18], China (n = 2) [19, 20], England (n = 1) [21], Germany (n = 3) [22,23,24], Greece (n = 2) [25, 26], Indonesia (n = 1) [27], Italy (n = 1) [28], Iran [29], Singapore (n = 1) [30], Portugal (n = 1) [31], Malaysia (n = 3) [32,33,34], Turkey (n = 1) [35], and USA (n = 3) [36,37,38] were studied.
According to World Bank classification of countries by income [39], fourteen studies were conducted in high income countries (Australia, England, Germany, Greece, Italy, Singapore, Portugal, and USA) and seven studies were conducted in upper-middle and low income countries (China, Indonesia, Malaysia, Turkey and Iran).
The studies mainly examined the age groups of 60 years and older, but in one study, the age group of 45 to 90 years was recruited [28]. In total, the present studies had totally 21,196 sample sizes that were performed in the general population. Short screening tools were used in all of the studies. The most widely used tool was the Mini Mental Status Examination (MMSE). The possibility of cognitive impairment was examined, so that in 17 studies (85%) [18,19,20,21,22,23,24, 26, 28,29,30,31,32,33,34,35,36], MMSE was used as a reference or index test. Due to the fact that the purpose of this study was to evaluate screening programs in the primary care ward, all studies were performed in primary care centers or family physician office. Screening was performed by family physicians or nurses or health care workers, and those whose cognitive status was positive at the first level (cognitive impairment), were referred to the secondary level (specialist clinics or psychiatrists or hospitals).
Screening tests
As an index test, all studies used short tools to diagnose cognitive disorders. MMSE were used in 14 studies [18,19,20,21,22,23, 25,26,27,28,29,30, 32,33,34, 38], General Practitioner Assessment of Cognition (GPCOG) in two studies [18, 26], Test Your Memory (TYM) in two study [26, 29], Early Dementia Questionnaire (EDQ) in two studies [32, 33], Ascertain Dementia 8-item (AD8) in one study [30], the Informant Questionnaire On Cognitive Decline in the Elderly (IQCODE) in one study [37], the Picture version of the Free and Cued Selective Reminding Test with Immediate Recall (pFCSRT + IR) in two studies [36, 38], Malay Version Rowland Universal Dementia Assessment Scale (M-RUDAS) in one study [24], a new screening method to support diagnosis of dementia (DemTect) in one study [34], and the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) in one study [27]. Also, as a reference test, 10 studies have used the agreement of psychiatrists or geriatricians [20, 21, 23, 25, 27, 28, 31, 35,36,37], one study [19] used CAMCOG, eight studies used MMSE [20, 24, 26, 29, 30, 33, 34, 37] and two studies used MOCA [19, 30] (Table 1).
EDQ and MMSE
The accuracy of EDQ diagnosis and its comparison with MMSE has been studied in two studies [32, 33]. In these studies, the sensitivity for EDQ was (0.669, 0.799) and the specificity was (0.477, 0.651). Positive and negative predictive values for EDQ were 23.5% and 93.2%, respectively. In one study, EDQ was compared to MMSE [32]. The prevalence of dementia was estimated 52.3% by using EDQ and 15.2% by using MMSE. Based on the findings of these two studies, EDQ has been introduced as a suitable alternative tool for MMSE for screening in primary care settings. Since this tool is tailored with the patients’ symptoms in a specific condition, so it has a high accuracy of diagnosis. Given the high negative predictive value of this test, the researchers believed that fewer cases of patients would be concealed from screening. Also, as this tool is more powerful than MMSE in diagnosing patients in the early stages of the disease, it has high power for detecting patients in early stage of cognitive disorders.
GPCOG and MMSE
The comparison of these two tests has been done in only one study [18]. In this study, the mean area under the curve (AUC) for GPCOG and MMSE was estimated to be 0.92 and 0.91%, respectively. However, there were no statistically significant differences between the two parameters. The sensitivity of GPCOG at the cut-off point of 11/10 and the sensitivity of MMSE at the cut-off point of 24/23 were estimated to be 0.79 and 0.51, respectively, which was also statistically significant. Researchers have reported better performance for GPCOG than MMSE despite spending less time for interviewing.
AD8, MMSE, and MOCA
The diagnostic features of the AD8, MMSE, and MOCA tools have been compared in a study [30] by using ROC curve. In order to evaluate the accuracy of diagnosis of these tools, a panel of experts has been used as the reference standard. Based on the findings, among people over 60 years with a cut-off point of 3.4, the sub-curve area criterion (AUC) for AD8 is equal to 0.97 with a 95% confidence interval (0.95—0.99), with sensitivity of 0.91, positive predictive value of 0.63, and negative predictive value of 0.97. For MOCA with a cut-off point of 16.17 AUC, sensitivity, specificity, positive predictive value and negative predictive value were 0.94 (0.92- 0.97), 0.84, 0.89, 0.56 and 0.97, respectively. The AD8 is superior to the MMSE and has similar performance to the MOCA. The AD8 showed similar performance among people over 75 years of age. In the Yang study [19], MMSE and MOCA were used among elderly population. Although the purpose of this study was not to compare the two tools, both instruments performed well in terms of evaluator agreement. In the Larner’s study, AUC of 0.64, sensitivity and specificity were reported 0.80 and 0.86, respectively, for MMSE (index test) compared to MOCA (reference test). Due to the low sensitivity of MMSE, researchers have not considered this tool suitable for use in screening in low prevalence areas for cognitive impairment and have introduced alternative tools such as MOCA with more efficiency. The researchers believed that, regardless of the cost of using MMSE and copyright considerations, it is not suitable for use in primary care in low prevalence conditions.
SIS and MMSE
The Short Screening Tool (SIS) [20] was derived from the MMSE tool. The different cutting points for the sensitivity of SIS have been reported. The most suitable cutting point is three, which has the sensitivity equal to 0.86, the specificity of 0.87, and AUC 95% CI: 0.93 (0.89–0.97). Researchers have found good validity for the SIS and believed that the summary of the SIS reduces the interview time and it is suitable for use among illiterate elderly population.
pFCSRT + IR and MMSE
Grobber [38] compared the diagnostic characteristics of two combined tools picture version of the Free and Cued Selective Reminding Test with Immediate Recall (pFCSRT) plus IR and MMSE. The AUC for pFCSRT + IR was greater than the MMSE (86% vs. 72%, P < 0.026). For diagnosis of dementia with the same specificity (81%), the sensitivity of MMSE was 48% (cut-off point less than 24) and the sensitivity of pFCSRT + AR was 70% (cut-off point less than 27). The sensitivity was reported 74% for both tests (cut point less than 28 for pFCSRT) and (cut point more than > 26) for MMSE. The specificity of pFCSRT was 75% and MMSE was 62%. The accuracy of pFCSRT was superior to MMSE. These tools take 10 to 15 min to be completed.
Pooled estimation of diagnostic accuracy of MMSE test
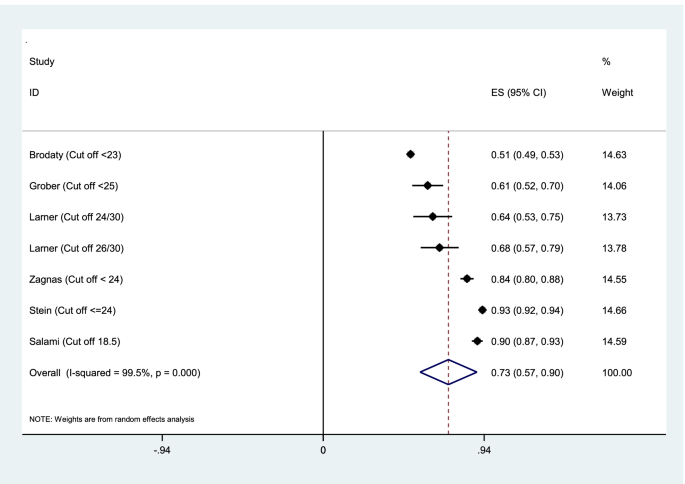
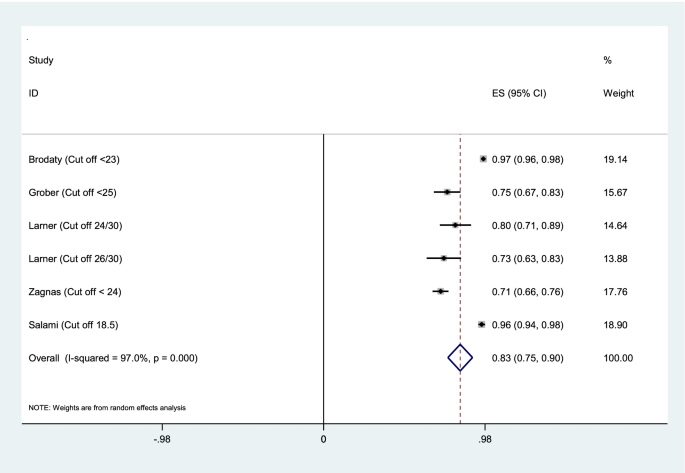
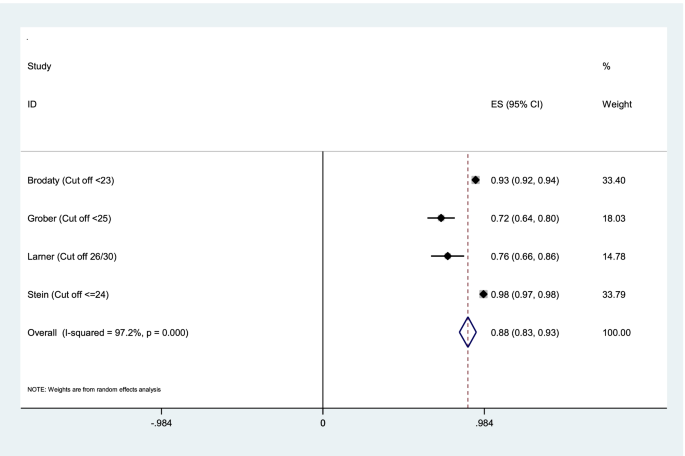
Aggregation of the values reported in seven studies for the sensitivity, specificity, and AUC of the MMSE test were used for meta-analysis. The cumulative sensitivity, specificity, and AUC analysis was conducted only for MMSE instrument. Due to the high heterogeneity in the studies, it was not possible to perform pooled analysis for all instruments. The diagnostic performance of the instruments used in the studies was systematically reviewed comparatively, the findings of which are presented in the following section. The random effect model for the MMSE showed the pooled sensitivity equal to 0.73 (95% CI 0.57–0.90) (Fig. 2), the pooled specificity equal to 0.83 (95% CI 0.75—0.90) (Fig. 3), and the pooled AUC 0.88 (95% CI 0.83–0.93) (Fig. 4).
The risk of bias in the studies is shown in Table 2. Also, the risk of bias and concern about the applicability of each domain of quality assessment studies based on QUADAS2 tool were shown in Fig. 5. Kapa coefficient score was estimated 0.908 (P < 0.0001) indicating strong agreement between two screening researchers.
Subgroup analysis
Table 3 shows the results based on the sensitivity, specificity, and AUC of MMSE according to subgroup analyses to explore the origin of the heterogeneity between the studies. The random-effects pooled estimation for sensitivity was 0.71 (95% CI 0.53–0.88; p < 0.001), for specificity was 0.81 (95% CI 0.67–0.95; p < 0.001), and for AUC was 0.73 (95% CI 0.67–0.80; p < 0.001) for participants aged 75 years and older. The higher random effect pooled estimation for sensitivity for the groups with respect to country’s income was for 0.91 low income countries (95% CI 0.89–0.94). The higher random effect pooled estimation for specificity was 0.97 (95% CI 0.96–0.97; p < 0.001) and for AUC was 0.97 (0.64–0.94; p < 0.001), respectively, for the groups with respect to sample size > 1000.
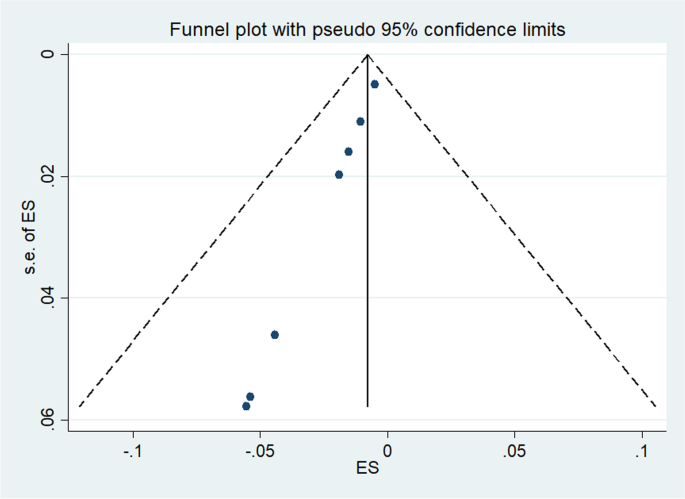
Publication bias
Publication bias was highlighted and confirmed by funnel plots. The funnel plots in Fig. 6 testing publication within diagnostic accuracy of MMSE tool. The graphical results point to asymmetry with a majority of the studies clustering to the left of the mean. Large studies are shown at the top of the graph, and smaller studies are shown at the bottom.
Discussion
The findings of the systematic review showed that the MMSE questionnaire is the most widely used tool and has been used as an indicator or reference test in most studies.
The findings of the present systematic review showed that there is insufficient evidence for community-based screening programs. The included final 21 studies in the systematic review also performed early detection of cognitive disorders on cross-sectional samples of the population and reported the accuracy of diagnosis of these tools. Of the 21 final studies, two studies [24, 27] recommended routine screening for cognitive disorders and three studies recommended against screening [19, 31, 36] that have pointed to the inability to implement community based screening, especially in low-income countries. Some substantial barriers of screening for cognitive disorders in low-income countries were highlighted such as limited resources for serving large population, insufficient training, and shortage of general physicians [19]. Another issues like living of most of older adults in remote rural areas or urban areas without having access the centers where offer routine screening tests [40]. These factors, along with other epidemiological and social factors like low educational level, low socio-economic status of older population, time and financial constraints, diagnostic uncertainty, stigma [35], and access of such people to health care centers contribute to the pause and challenging of screening programs in low income countries [5, 41] However, Koch et al. [35] in a rapid appraisal of barriers to the diagnosis of cognitive disorders and dementia stated that health care systems were accountable for the several mentioned barriers [42]. Eichler [24] and Pandahita [27] agreements for performing routine screening were the high percentage of undiagnosed patients in primary care settings and also the fact that the proposed screening test did not provide enough information about the feasibility of screening. Therefore, these two studies would not be recognized sufficient evidence for screening cognitive disorders. The findings are in line with the recommendations of the US Preventive Services Committee Task Force (USPSTF) in 2003, 2011, 2014 and most recently in 2020. The committee believes that there was no evidence to prove the screening program could improve the current care process [12]. The Alzheimer's Association of the United States cites this evidence and recommends the inclusion of an early detection program for cognitive disorders in the annual geriatric visits [5, 43]. Iliffe et al. [43] stated that they were not able to identify an advantage for routine screening test, but they considered the possibility of early detection in primary care. Therefore, the program for diagnosing cognitive disorders is beyond the informal observation by a physician and is an ongoing process that is diagnosed during various stages of senile disorder. Counselling and interviewing before and after the diagnosis of the disorder is an important part of the diagnosis process and the use of caregivers and elderly people would be effective in diagnosing the disease [43]. The National Institute for Clinical Excellence (NICE) and the UK National Health System's advisory did not consider routine screening to be cost-effectiveness in their recommendations in 2006.
More than 12 different tools have been used in the final studies. MMSE tool is the most widely used and common tool in this field. Comparison of instruments showed that IQCODE, GPCOG, AD8, MOCA, PFCSRT + IR and EDQ instruments had detection power equal to or higher than MMSE. Even the MMSE short tool had good diagnostic performance. The present finding shows that the above tools can replace MMSE in the diagnosis of cognitive disorders and dementia. In addition, MMSE because of being long, not free and is biased towards the literacy level of the participants, the Alzheimer's Association has introduced six criteria for selecting the right tool, including evaluation time of less than 5 min, validation evidence in primary care, usability by non-medical staff, appropriate psychometric properties, insensitivity to literacy, language and culture bias, and it’s free availability. The Alzheimer's Association based on the findings of the previously published systematic review studies [44,45,46,47] showed appropriate tools for assessing patients' cognition, including GPCOG, Mini-Cog, and MIS, and interviews with IQCODE, AD8, and GPCOG caregivers. Our systematic review findings are also in line with the recommendations of the Alzheimer's Association. MOCA, IQCODE, GPCOG and MMSE instruments have also been validated in Iran [29, 48, 49], but participants were recruited from the general population setting rather than the primary care units. Consistent with our study, a review study on brief cognitive screening instruments found that MMSE is the most frequently used cognitive screening tool in the community and primary care. The study also highlighted that mini cognition (Mini-cog), memory impairment screen (MIS), and the general practitioner assessment of cognition (GPCOG) were beneficial in primary care setting and recommended for use [47]. Based on the findings, practicality, psychometric properties of instruments, validation in a community, general population, or referring people for primary care setting, as well as utility, efficacy, and administration time were major criteria for implementing the cognitive screening instruments in primary care and community programs especially in low income countries.
Limitations
The available studies were carried out in the variety of high and middle income countries. There was no study in low level country to clarify the advantages or disadvantages of screening programs in these countries. Overall, additional researches are needed to identify the best screening tool in low income countries.
Conclusion
There was insufficient evidence for routine and general screening to identify cognitive disorders. However, due to the high incidence of undiagnosed patients and the benefits of early diagnosis in caregiver management, the integration of early diagnosis into annual or periodic geriatric care programs has been used in most high-income countries. The use of non-medical staff in the initial assessment can be suggested as a suitable option, especially in countries that face a shortage of medical staff. Although MMSE is the most widely used diagnostic tool, according to the current systematic review, MOCA, GPCOG and MIS tools can be used to evaluate patients and IQCODE, AD8 and GPCOG tools can be used to evaluate their caregivers with equal or better performance than MMSE.
Availability of data and material
The data collection tools and datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AQT:
-
A Quick Test of Cognitive Speed,
- RCS-T:
-
Rapid Recognition Screening
- SIS:
-
Six-Item Screener
- PRISMA:
-
Preferred report items for systematic review and meta-analysis
- QUADAS:
-
Quality Assessment of Diagnostic Accuracy Studies-2
- AWV:
-
Annual wellness visit
- MMSE:
-
Mini–mental state examination
- GPCOG:
-
General practitioner assessment of cognition
- AD8:
-
Ascertain dementia 8-item
- TYM:
-
Test your memory
- EDQ:
-
Early Dementia Questionnaire
- IQCODE, pFCSRT + IR:
-
The picture version of the Free and Cued Selective Reminding Test with Immediate
- M-RUDAS:
-
The Recall Malay Version Rowland Universal Dementia Assessment Scale
- DemTect:
-
A new screening method to support diagnosis of dementia
References
Pink Jta, O'Brien Jpooap, Robinson Lpopc, ageing, Longson DclpcoGC, on behalf of the Guideline C: Guidelines: Dementia: assessment, management and support: summary of updated NICE guidance. BMJ. 2018;23(361):k2438.
Gauthier S R-NP, Morais JA, & Webster C. : World Alzheimer Report 2021: Journey through the diagnosis of dementia. In. London, England: Alzheimer’s Disease International.; 2021.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62.
Santos CY, Snyder PJ, Wu W-C, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Dement (Amst). 2017;7:69–87.
Cordell CB, Borson S, Boustani M, Chodosh J, Reuben D, Verghese J, Thies W, Fried LB. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9(2):141–50.
Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306–14.
Bianchetti A, Ferrara N, Padovani A, Scarpini E, Trabucchi M, Maggi S. Timely detection of mild cognitive impairment in Italy: an expert opinion. Journal of Alzheimer’s disease : JAD. 2019;68(4):1401–14.
Woods B, Arosio F, Diaz A, Gove D, Holmerová I, Kinnaird L, Mátlová M, Okkonen E, Possenti M, Roberts J, et al. Timely diagnosis of dementia? family carers’ experiences in 5 European countries. Int J Geriatr Psychiatry. 2019;34(1):114–21.
Dhedhi SA, Swinglehurst D, Russell J. “Timely” diagnosis of dementia: what does it mean? A narrative analysis of GPs’ accounts. BMJ open. 2014;4(3):e004439.
Werner P, Goldstein D, Karpas DS, Chan L, Lai C. Help-seeking for dementia: a systematic review of the literature. Alzheimer Dis Assoc Disord. 2014;28(4):299–310.
Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR Jr. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–55.
Patnode CD, Perdue LA, Rossom RC, Rushkin MC, Redmond N, Thomas RG, Lin JS. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(8):764–85.
Rosenbaum S. The patient protection and affordable care act: implications for public health policy and practice. Public Health Rep. 2011;126(1):130–5.
National Collaborating Centre for Mental H: National Institute for Health and Clinical Excellence: Guidance. In: Dementia: A NICE-SCIE Guideline on Supporting People With Dementia and Their Carers in Health and Social Care. edn. Leicester (UK): British Psychological Society Copyright © 2007, The British Psychological Society & The Royal College of Psychiatrists.; 2007.
Pelegrini LNC, Mota GMP, Ramos CF, Jesus E, Vale FAC. Diagnosing dementia and cognitive dysfunction in the elderly in primary health care: a systematic review. Dement Neuropsychol. 2019;13(2):144–53.
Lin JS, O’Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(9):601–12.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Brodaty H, Connors MH, Loy C, Teixeira-Pinto A, Stocks N, Gunn J, Mate KE, Pond CD. Screening for dementia in primary care: a comparison of the GPCOG and the MMSE. Dement Geriatr Cogn Disord. 2016;42(5–6):323–30.
Yang Y, Xiao LD, Deng L, Wang Y, Li M, Ullah S. Nurse-led cognitive screening model for older adults in primary care. Geriatr Gerontol Int. 2015;15(6):721–8.
Xue J, Chiu HFK, Liang J, Zhu T, Jiang Y, Chen S. Validation of the Six-Item Screener to screen for cognitive impairment in primary care settings in China. Aging Ment Health. 2018;22(4):453–7.
Larner AJ. Mini-Mental State Examination: diagnostic test accuracy study in primary care referrals. Neurodegenerative disease management. 2018;8(5):301–5.
Stein J, Luppa M, Kaduszkiewicz H, Eisele M, Weyerer S, Werle J, Bickel H, Mösch E, Wiese B, Prokein J, et al. Is the Short Form of the Mini-Mental State Examination (MMSE) a better screening instrument for dementia in older primary care patients than the original MMSE? Results of the German study on ageing, cognition, and dementia in primary care patients (AgeCoDe). Psychol Assess. 2015;27(3):895–904.
Thyrian JR, Eichler T, Michalowsky B, Wucherer D, Reimann M, Hertel J, Richter S, Dreier A, Hoffmann W. Community-dwelling people screened positive for dementia in primary care: a comprehensive, multivariate descriptive analysis using data from the DelpHi-Study. Journal of Alzheimer’s disease : JAD. 2016;52(2):609–17.
Eichler T, Thyrian JR, Hertel J, Michalowsky B, Wucherer D, Dreier A, Kilimann I, Teipel S, Hoffmann W. Rates of formal diagnosis of dementia in primary care: the effect of screening. Alzheimers Dement (Amst). 2015;1(1):87–93.
Zaganas IV, Simos P, Basta M, Kapetanaki S, Panagiotakis S, Koutentaki I, Fountoulakis N, Bertsias A, Duijker G, Tziraki C. The Cretan aging cohort: cohort description and burden of dementia and mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2019;34(1):23–33.
Iatraki E, Simos PG, Bertsias A, Duijker G, Zaganas I, Tziraki C, Vgontzas AN, Lionis C. Cognitive screening tools for primary care settings: examining the “Test Your Memory” and “General Practitioner assessment of Cognition” tools in a rural aging population in Greece. Eur J Gen Pract. 2017;23(1):171–8.
Pandhita SG, Sutrisna B, Wibowo S, Adisasmita AC, Rahardjo TBW, Amir N, Rustika R, Kosen S, Syarif S, Wreksoatmodjo BR. Decision tree clinical algorithm for screening of mild cognitive impairment in the Elderly in Primary Health Care: Development, Test of Accuracy, and Time-Effectiveness Analysis. Neuroepidemiology. 2020;54(3):243–50.
Petrazzuoli F, Palmqvist S, Thulesius H, Buono N, Pirrotta E, Cuffari A, Cambielli M, D'Urso M, Farinaro C, Chiumeo F, Marsala V, Wiig EH. A Quick Test of Cognitive Speed: norm-referenced criteria for 121 Italian adults aged 45 to 90 years. Int Psychogeriatr. 2014:1–8. https://doi.org/10.1017/S1041610214000787. Epub ahead of print.
Salami M, Alinaghipour A, Daneshvar R, Hamidi GA, Agahi A, Soheili M, Akbari H, Esmaeili Taba SM. Adapted MMSE and TYM cognitive tests: how much powerful in screening for Alzheimer’s disease in Iranian people. Aging Ment Health. 2020;24(6):1010–7.
Chan QL, Xu X, Shaik MA, Chong SST, Hui RJY, Chen CL-H, Dong Y. Clinical utility of the informant AD8 as a dementia case finding instrument in primary healthcare. J Alzheimers Dis. 2016;49(1):121–7.
Teixeira L, Dos Santos PM, Alves S, Azevedo MJ, Duarte MG, Leuschner A, Paúl C. Screening of dementia in Portuguese primary care: Methodology, assessment tools, and main results. Front Med. 2017;13(4):197.
Arabi Z, Aziz NA, Abdul Aziz AF, Razali R, Wan Puteh SE. Early Dementia Questionnaire (EDQ): a new screening instrument for early dementia in primary care practice. BMC Fam Pract. 2013;14:49.
Arabi Z, Syed Abdul Rahman SA, Hazmi H, Hamdin N. Reliability and construct validity of the Early Dementia Questionnaire (EDQ). BMC Geriatr. 2016;16(1):202.
Shaaban J, Aziz AA, Abdullah Z, Razak AA. Validation of the malay version of rowland universal dementia assessment scale (m-rudas) among elderly attending primary care clinic. International Medical Journal. 2013;20(5):555–8.
KocOkudur S, Dokuzlar O, Kaya D, Soysal P, Isik AT. Triple test plus rapid cognitive screening test: a combination of clinical signs and a tool for cognitive assessment in older adults. Diagnostics. 2019;9(3):97.
Grober E, Ehrlich AR, Troche Y, Hahn S, Lipton RB. Screening older Latinos for dementia in the primary care setting. J Int Neuropsychol Soc. 2014;20(8):848–55.
Grober E, Mowrey WB, Ehrlich AR, Mabie P, Hahn S, Lipton RB. Two-stage screening for early dementia in primary care. J Clin Exp Neuropsychol. 2016;38(9):1038–49.
Grober E, Wakefield D, Ehrlich AR, Mabie P, Lipton RB. Identifying memory impairment and early dementia in primary care. Alzheimers Dement (Amst). 2017;6:188–95.
https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html Accessed 14 Jun
Cullen B, O’Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78(8):790–9.
Brucki SMD, Nitrini R. Subjective memory impairment in a rural population with low education in the Amazon rainforest: an exploratory study. Int Psychogeriatr. 2008;21(1):164–71.
Tabrizi JS, HaghGoshayie E, Doshmangir L, Yousefi M. New public management in Iran’s health complex: a management framework for primary health care system. Primary health care research & development. 2018;19(3):264–76.
Iliffe S, Robinson L, Brayne C, Goodman C, Rait G, Manthorpe J, Ashley P. Primary care and dementia: 1. diagnosis, screening and disclosure. Int J Geriatr Psychiatry. 2009;24(9):895–901.
Setter SM, Neumiller JJ, Weeks DL, Borson S, Scanlan JM, Sonnett TE. Screening for undiagnosed cognitive impairment in homebound older adults. The Consultant pharmacist : the journal of the American Society of Consultant Pharmacists. 2009;24(4):299–305.
Holsinger T, Boustani M, Abbot D, Williams JW. Acceptability of dementia screening in primary care patients. Int J Geriatr Psychiatry. 2011;26(4):373–9.
Milne A, Culverwell A, Guss R, Tuppen J, Whelton R. Screening for dementia in primary care: a review of the use, efficacy and quality of measures. Int Psychogeriatr. 2008;20(5):911–26.
Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry. 2010;25(2):111–20.
Badrkhahan SZ, Sikaroodi H, Sharifi F, Kouti L, Noroozian M. Validity and reliability of the Persian version of the Montreal Cognitive Assessment (MoCA-P) scale among subjects with Parkinson’s disease. Appl Neuropsychol Adult. 2020;27(5):431–9.
Foroughan M, Jafari Z, Farahani I, Rashedi V. Validity and Reliability of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Preliminary Findings Among the Older Population of Iran. GeroPsych. 2019;32:145–51.
Acknowledgements
We acknowledge the contributions of Tabriz University of Medical Sciences, Tabriz, Iran for providing facilities to the study.
Funding
Tabriz University of Medical Sciences provided facilities.
Author information
Authors and Affiliations
Contributions
LJ is responsible for the study design. LJ and LK did the analyses. LJ and AMA were responsible for data interpretation. LK, AMA, HSB, MF helped in the study design and data gathering, LK and AMA helped in the drafting of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study received ethical approval from the Ethics Committee of Tabriz University of Medical Sciences (NO: IR.TBZMED.REC. 1398. 139).
Consent for publication
Not applicable
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Karimi, L., Mahboub–Ahari, A., Jahangiry, L. et al. A systematic review and meta-analysis of studies on screening for mild cognitive impairment in primary healthcare. BMC Psychiatry 22, 97 (2022). https://doi.org/10.1186/s12888-022-03730-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-022-03730-8